FEV1: The Vital Lung Function Metric Tied to Longevity
Forced Expiratory Volume in 1 Second, or FEV₁, is emerging as a powerful biomarker for healthy aging and lifespan. In this article, we'll explore what FEV₁ measures, why it strongly predicts longevity, how it changes with age and habits, its links to chronic diseases and biological aging, and science-backed ways to improve it. By the end, you'll understand why maintaining your FEV₁ may help you live not just longer, but healthier – and how you can track your own aging with tools like the TruAge biological age test.
Scientifically Reviewed by
Dr. Olena Husak, PhD
What is FEV₁?
FEV₁ stands for Forced Expiratory Volume in 1 second. It is the maximal volume of air you can forcefully exhale from fully inflated lungs in one second[1]. In practical terms, imagine taking a deep breath in and then blowing out as hard and fast as possible – the amount of air expelled in the first one second is your FEV₁. This single number captures a lot about your lung function. A high FEV₁ indicates open, elastic airways that can move air quickly, whereas a low FEV₁ suggests narrowed or stiff airways that limit airflow.
FEV₁ primarily reflects lung capacity and airway health. It depends on lung size (which is related to your height, sex, and genetics) and how freely air flows through your bronchi and bronchioles. Clinicians often measure FEV₁ using spirometry – you breathe into a specialized sensor – as part of pulmonary function testing. Because it correlates with how well you can supply your body with oxygen during each breath, FEV₁ is a core indicator of overall respiratory function. Healthy young adults typically have high FEV₁ values (several liters of air). If lung disease (like asthma or chronic obstructive pulmonary disease, COPD) narrows the airways, the FEV₁ drops. Even in people without diagnosed lung conditions, a below-normal FEV₁ can signal reduced pulmonary reserve. In short, FEV₁ measures the vital capacity and efficiency of your lungs – a foundation for physical endurance and well-being[2].
Why FEV₁ Matters for Longevity
Mounting research shows that FEV₁ is not just about the lungs – it's a window into your future health and lifespan. In epidemiology studies tracking thousands of adults over decades, baseline FEV₁ emerges as one of the strongest predictors of mortality risk from all causes. People with higher FEV₁ tend to live significantly longer than those with low values [3][4]. Importantly, this prediction holds even after accounting for factors like smoking, medical conditions, and demographics [5]. In fact, some scientists have called lung function measured by FEV₁ "a biomarker of aging that integrates the cumulative damage of life exposures" on the body.
Large cohort studies in Europe and globally illustrate this vividly. For example, the international PURE study (Prospective Urban Rural Epidemiology) followed over 126,000 adults in 17 countries. It found a graded relationship between lower FEV₁ (standardized for age, sex, height, etc.) and higher risk of death and serious illness [3]. Even individuals with mildly reduced FEV₁ – still within what clinicians consider the "normal" range – had elevated risks compared to those with the highest lung function [3]. The PURE investigators concluded that "FEV₁ is an independent and generalisable predictor of mortality, cardiovascular disease, and respiratory hospitalization, even across the clinically normal range" [3]. Similarly, a study of 25,000 adults in four European countries reported that even a modest FEV₁ reduction (just 10–15% below expected) was associated with ~25% higher risk of death, while very low FEV₁ (severe impairment) more than tripled the risk [4]. Notably, the link persisted in never-smokers, indicating FEV₁ isn't just a proxy for smoking but a robust marker on its own [5].
Why would lung function be such a powerful longevity indicator? One reason is that FEV₁ integrates many aspects of health: respiratory muscle strength, airway integrity, and even immune health (lungs interface with environmental pathogens and pollutants). A higher FEV₁ may reflect a life relatively free from the cumulative insults (like smoking, air pollution, chronic infections, poor nutrition) that also contribute to aging elsewhere in the body. Conversely, a low FEV₁ can result from chronic inflammation and oxidative stress that accelerate aging in multiple organs. [6]There's also a practical aspect: good lung function supports exercise capacity and activity levels, which in turn protect against cardiovascular disease, metabolic disease, and frailty. In essence, FEV₁ is a sentinel of vitality – if your lungs are aging slowly, chances are the rest of you is too.
What Are Healthy FEV₁ Values by Age and Sex?
Like many health metrics, "normal" FEV₁ depends on your age, sex, and size. Young adults have the highest values, which then decline gradually with age. Men usually have higher FEV₁ than women because on average they have larger lungs. Reference charts (such as the Global Lung Initiative 2012 equations used in Europe) account for these factors to give an expected FEV₁ for a healthy person of a given age, sex, height, and ethnicity[7]. Doctors often express your result as a percent of the predicted value. For instance, if your measured FEV₁ is 3.0 liters but the reference for a 30-year-old man of your height is 4.0 L, then you are at 75% of predicted. Generally, above ~80% of predicted is considered normal, while values below ~80% (around the lowest 5th percentile) may indicate impairment[7].
Peak lung function is typically reached in one's 20s. In a healthy 30-year-old male, FEV₁ might be roughly 4 liters (for a female ~3 liters, depending on height). From about age 30 onward, FEV₁ naturally declines as part of aging, even in never-smokers. How fast it declines is crucial. In one European cohort, never-smoking men lost about 31 mL per year and women about 27 mL per year of FEV₁ on average[19]. This means by age 80, a never-smoking man might have only ~70–80% of the FEV₁ he had at 30. By contrast, smoking dramatically accelerates this decline. Long-term smokers can lose FEV₁ at double the rate of non-smokers or more[10]. For example, a heavy smoker might lose ~45 mL each year, causing an earlier and steeper drop in lung capacity.
Because of these patterns, healthy FEV₁ values by age follow a percentile curve. As an example, an FEV₁ of 2.5 L might be normal for an 80-year-old man (around 100% of predicted at that age), but the same 2.5 L in a 40-year-old man would be only ~60% of predicted (very low for that age). European reference data provide percentiles: if your FEV₁ is at the 50th percentile, half of healthy people your age have a lower value and half higher; if you're below the 5th percentile, 95% of peers breathe better than you. Unsurprisingly, lifelong smokers tend to be at lower percentiles. For instance, a 50-year-old female smoker might have the lung function of a 65-year-old nonsmoking female[19][10]. The encouraging flip side is that quitting smoking helps: someone who stops smoking will thereafter lose lung function at roughly the normal rate, preserving more FEV₁ into older age[11].
In summary, a "healthy" FEV₁ for your profile is one in the upper ranges for your age/sex – ideally above 80–100% of the predicted value. If your FEV₁ is below 80% predicted, it falls in the low end of the distribution where risks start to rise. Tracking where you stand (percentile-wise) as you get older is a smart way to gauge if your lungs are aging faster or slower than expected.
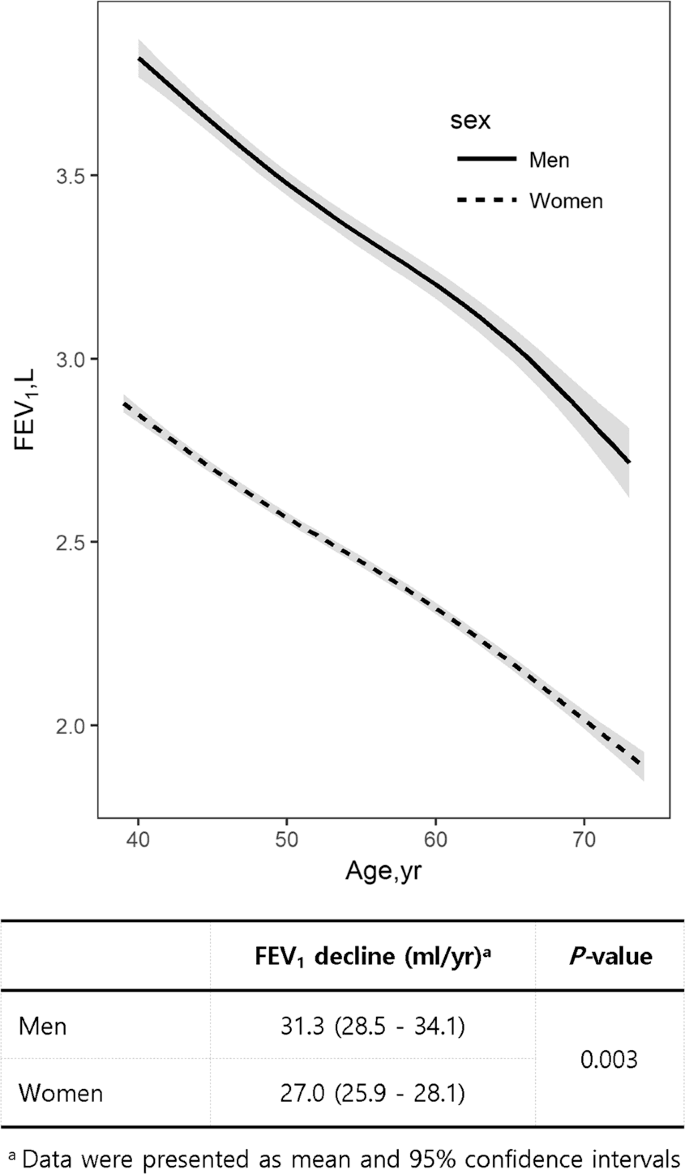 FEV₁ declines with age in both men and women, with men showing a slightly faster decline. Data are mean and 95% confidence intervals. Adapted from published cohort studies. [19]
FEV₁ declines with age in both men and women, with men showing a slightly faster decline. Data are mean and 95% confidence intervals. Adapted from published cohort studies. [19]How Is FEV₁ Measured?

Clinical Spirometry
Spirometry is the gold-standard test to measure FEV₁[1]. In a clinical spirometry test, you inhale deeply then exhale into a mouthpiece "as hard and fast as possible" until your lungs are empty. The spirometer device records the volume of air exhaled over time. From this curve, the FEV₁ is determined as the volume exhaled in the first 1.0 second. For accuracy, the test is repeated several times with coaching to ensure you truly gave a maximal effort. Spirometry also measures FVC (forced vital capacity, total air exhaled) and the ratio FEV₁/FVC, but FEV₁ itself is often the key number. Testing is typically done in hospitals, clinics, or lung function labs by trained technicians following standard protocols (like ATS/ERS guidelines) to get reliable results[1][1].

At-Home Digital Spirometers
Traditionally, you needed to visit a clinic for spirometry, but technology is changing that. At-home digital spirometers have become available that let you measure FEV₁ on your own. Many of these are small handheld devices (some the size of an inhaler) with a mouthpiece and a sensor, connecting to a smartphone app via Bluetooth. Studies have found that quality portable spirometers – for example the "Air Next" device – can produce FEV₁ readings comparable to clinical lab spirometry when used correctly[14][15]. Home spirometry gained popularity during the COVID-19 pandemic when clinic access was limited, and research confirms that for patients with fairly stable lung disease or in healthy users, home measurements are feasible and reasonably accurate[15][15]. However, using them requires good technique and consistency. Unsupervised home tests sometimes underestimate FEV₁ slightly compared to supervised tests[15], likely due to suboptimal effort or coaching at home.
Overall, spirometry remains the definitive test for FEV₁[1]. At-home options are supplementing, not replacing, formal testing. If an at-home device flags a low FEV₁ or a big drop, you'd want to follow up with a professional test. The good news is that measuring this longevity-linked metric is getting easier – you might not need to wait for your next physical; you could check your lung age today in your living room.
FEV₁ and Chronic Disease Risks
Chronic Obstructive Pulmonary Disease (COPD)
Cardiovascular Disease
Metabolic Health and Diabetes
There is growing evidence that poor lung function and metabolic disorders go hand in hand. Studies show that people with type 2 diabetes tend to have slightly lower FEV₁ on average, even after accounting for smoking[22]. Prospective research suggests low FEV₁ may precede diabetes or make it more likely. For instance, a European study reported that individuals with FEV₁ below ~80% predicted had higher odds of developing insulin resistance and diabetes over time[23][23]. The link might be mediated by inflammation – pro-inflammatory cytokines can reduce lung function and interfere with insulin action[24][25]. Additionally, shared risk factors like abdominal obesity and physical inactivity contribute to both. While the connection isn't as widely recognized as that with heart disease, it appears the lungs and pancreas are indirectly connected. In any case, maintaining good aerobic fitness (which correlates with higher FEV₁) is known to improve glucose metabolism. Conversely, if someone's FEV₁ is low for their age, it might prompt checking for metabolic issues too.
Cognitive Decline and Dementia
One of the most intriguing associations is between midlife lung function and brain health in later life. Long-running studies have found that people with poorer lung function in their 40s and 50s are more likely to experience cognitive decline or be diagnosed with dementia in old age[26][27]. A 27,000-person study by Kaiser Permanente researchers showed each liter lower FEV₁ at midlife was linked to a ~13% higher risk of dementia over the next ~25 years[28]. Those in the lowest FEV₁ quintile (bottom 20%) had about 24–28% greater dementia incidence than those in the highest quintile[28]. Notably, this analysis adjusted for smoking and vascular diseases, suggesting an independent lung-brain connection.
Summary & Systemic Impact
Possible explanations: chronic hypoxia (low oxygen) from subpar lung function could gradually impair the brain, or common risk factors (like pollution exposure) could contribute to both lung and neural aging[28][29]. Another hypothesis is that lifelong fitness and education (which preserve lung function) also build cognitive reserve[31]. Regardless of cause, FEV₁ seems to reflect system-wide aging, including the brain. Protecting your lungs might literally help keep your mind sharp longer.
In summary, a low FEV₁ is a multi-system risk marker. It doesn't mean you will definitely get these diseases, but it flags a higher probability. Doctors sometimes incorporate lung function into risk scores for overall mortality and morbidity[32]. The converse is also true: maintaining a high FEV₁ (for your age) is protective and is often seen in people who reach very old ages in good health. This wide-ranging impact is why some call FEV₁ a measure of "biological age." It captures the cumulative wear-and-tear on critical systems – respiratory, cardiovascular, metabolic, and even cognitive.
FEV₁ and DNA Methylation Aging Clocks
Modern longevity research has given us epigenetic clocks – blood tests that measure DNA methylation patterns to estimate biological age. Interestingly, what these cutting-edge clocks reflect often aligns with traditional measures like FEV₁. After all, an epigenetic age acceleration means your body's systems (heart, lungs, brain, etc.) are aging faster than expected. Because FEV₁ so directly indicates system integrity, it tends to correlate with these biological age measures.
For instance, the DunedinPACE clock (Pace of Aging Computed from the Epigenome) was developed by tracking a cohort's physiological changes, including lung function, over decades [36]. In the original Dunedin Study, researchers measured the decline in FEV₁, along with kidney function, immune biomarkers, gum health and more, in participants from their 20s into midlife. These data fed into DunedinPACE, a DNA methylation algorithm that outputs a "speed of aging" – e.g. 1.0 means you're aging biologically one year for each chronological year, 1.1 means 1.1 years of aging per year (faster aging), 0.9 means slower aging. People with faster DunedinPACE scores were observed to experience greater declines in physical functions like balance, motor coordination, and lung capacity by midlife [36]. In other words, those aging faster biologically showed lower FEV₁ (and greater FEV₁ decline) relative to their peers aging more slowly. This confirms that FEV₁ indeed registers in our biological aging.
Another set of new clocks, developed by commercial and academic collaborations, explicitly connects to organ-specific aging. The OMICm Age algorithm is a next-generation epigenetic clock that integrates multi-omic data (including metabolomic and proteomic markers) to predict biological age. According to reports, OMICm Age showed the highest correlation to health outcomes (like chronic disease incidence) compared to earlier clocks [37]. While specific details of OMICm Age are proprietary, it likely captures signals from the lungs as well. The reason is many blood biomarkers in OMICm (for example, markers of inflammation or collagen turnover) can reflect lung tissue aging and fibrosis, which influence FEV₁. Indeed, OMICm Age was able to predict 10-year mortality with ~87% accuracy in a test cohort [37], suggesting it effectively senses critical organ aging – including the lungs.
Perhaps the most fascinating is the new SYMPHONY Age clock, created with researchers at Yale. Symphony Age doesn't just give one age; it aims to estimate the biological age of 11 different organ systems (like a symphony of your body's parts) [38]. One of those systems is the pulmonary system. In essence, Symphony Age deconstructs your epigenome to tell how old your lungs are (and your heart, kidneys, liver, etc.) relative to your chronological age. If your Symphony lung-age is older than your actual age, it likely means your FEV₁ is lower than normal for your age (since lung capacity is a major aspect of lung aging). Conversely, if your lung-age comes back younger, that implies above-average FEV₁ [39]. These advanced tools thereby reinforce the importance of FEV₁: it's such a fundamental indicator that it's being reverse-engineered from DNA methylation patterns.
To put it simply, FEV₁ intersects with biological aging measures in two ways. First, declines in FEV₁ contribute to calculated aging metrics (like DunedinPACE) as one of the manifestations of aging. Second, even independent of how clocks are built, an individual with a low FEV₁ tends to have an older biological age. This was observed with earlier clocks like Horvath's and Hannum's as well – people with lung function impairment often show accelerated epigenetic aging (likely due to high oxidative stress and inflammation). On the bright side, improving your lung health might slow down these epigenetic clocks. In a caloric restriction trial, participants who slowed their biological aging also had better cardiorespiratory fitness than controls [40], hinting that lung function preservation was part of the slowed aging package.
In summary, longevity science and epigenetic clocks are validating what FEV₁ has hinted at all along: aging is a whole-body phenomenon, and the lungs are a central player. Whether through DunedinPACE's "speedometer" or Symphony Age's organ-specific report, your lung function is embedded in your biological age. This convergence is exciting – it means we can use accessible measures like FEV₁ alongside cutting-edge blood tests to get a comprehensive picture of our aging process.
How to Improve FEV₁ (Scientifically)
Aerobic Exercise and Fitness: Regular cardiovascular exercise (such as brisk walking, running, cycling, swimming) can modestly increase lung capacity and strengthen respiratory muscles. Endurance athletes often have FEV₁ values 5–15% higher than sedentary individuals, because exercise demands deep breathing and full lung inflation. Even starting exercise later in life helps: a study in older adults found those who stayed active had a much slower decline in FEV₁ over ~10 years[33][34]. Exercise improves the efficiency of oxygen uptake and can reduce inflammation, indirectly supporting better lung function.
Pulmonary Rehabilitation and Breathing Exercises: In people who already have reduced lung function or COPD, formal pulmonary rehab programs can improve FEV₁ or at least slow its decline. These programs combine supervised exercise with breathing techniques (like diaphragmatic breathing and pursed-lip breathing) to help patients use their lung capacity more fully. A meta-analysis in COPD patients showed that rehab led to small but significant increases in FEV₁, especially when started early in moderate disease[35]. For generally healthy people, deep-breathing exercises (such as yoga pranayama or using an incentive spirometer device) might help maintain chest wall flexibility and respiratory muscle strength[35]. Though the FEV₁ gains from breathing exercises alone are usually minor, they can improve your comfort in taking truly deep breaths, which translates to better test performance.
Smoking Cessation (and Avoidance of Secondhand Smoke): If you smoke, quitting is the single most impactful step for your FEV₁. Smoking damages airways and accelerates FEV₁ decline dramatically[33]. The classic Fletcher-Peto study showed that smokers who quit in midlife had a subsequent FEV₁ decline slope almost as flat as never-smokers – essentially preserving what lung function they had left[11]. Some ex-smokers even see a slight recovery in FEV₁ in the first year after quitting as airway inflammation subsides. Avoiding secondhand smoke and environmental dust/fumes is similarly important; chronic exposure to air pollutants has been shown to impair lung growth in children and expedite lung aging in adults.
Nutrition and Antioxidants: The lungs are exposed to a lot of oxidative stress. Diets rich in antioxidants and anti-inflammatory nutrients can theoretically protect lung function. Studies have linked higher intake of fruits (especially those high in vitamin C and flavonoids) with better FEV₁ and slower decline. For example, a large study in Wales found that men who ate fresh fruit daily had a significantly smaller FEV₁ decline over 5 years than those who rarely ate fruit[34]. Omega-3 fatty acids (from fish or flaxseed) may also have lung benefits via reducing airway inflammation. Furthermore, maintaining a healthy weight prevents the restriction on lung volume that comes with obesity (excess abdominal fat can limit diaphragmatic movement, effectively lowering FEV₁).
Manage Respiratory Conditions Proactively: If you have asthma or allergies, controlling them can preserve your lung function. Untreated chronic asthma (with ongoing inflammation and bronchoconstriction) can lead to airway remodeling that reduces FEV₁ over time. Similarly, prompt treatment of respiratory infections (like not letting a bad pneumonia scar your lungs) is important.
Environmental Interventions: Clean air is key for healthy lungs. If you can, improve the air quality in spaces where you spend a lot of time. For instance, reduce indoor pollutants (ventilate when cooking, avoid strong chemical fumes, don't burn wood or coal indoors without proper ventilation). Occupational exposures are critical too – if you work around dust, vapors, or combustion fumes, use protective equipment. Studies on occupational cohorts (like coal miners or construction workers) show those who used masks and had dust controls in place had better preserved FEV₁ than those who didn't.
Short-term vs long-term improvements: In healthy individuals, dramatic short-term jumps in FEV₁ are uncommon unless there was an unrecognized issue (e.g., using an bronchodilator inhaler can bump up FEV₁ if you had mild airway narrowing). Most of the above strategies work gradually or prevent further loss. You might not see your FEV₁ go up 20% in a year, but you could prevent it from falling 20% in the next decade, which is a huge win. Think of it like retirement savings – building lung reserves when you're younger and maintaining them pays off later. And if your FEV₁ is already low, these approaches can slow any further decline and sometimes yield small gains (for example, an COPD patient in rehab might improve FEV₁ by 5–10% and importantly improve symptoms).
In essence, treat your lungs as a lifelong investment. Regular exercise is like depositing into your "lung function bank," whereas smoking is like making large withdrawals. Nutrition and clean air provide the maintenance that keeps your lung tissue healthy at the cellular level. The science overwhelmingly supports that by following these habits, you can add not just years to your life, but life to your years – with FEV₁ as a measurable indicator of that progress.
Ready to Take Control of Your Lung Health?
Now that you understand how FEV₁ impacts your longevity, it's time to take action. Access TruAge epigenetic testing to get a comprehensive view of your biological age, including your lung age, and FEV₁ so you can track and improve your healthspan.
Join 97 early access testers who are already tracking their pace of aging
References
- 1.ARTP statement on pulmonary function testing 2020Sylvester KP, Clayton N, Cliff I, Hepple M, Kendrick A, Kirkby J, et al. BMJ Open Respir Res 2020;7(1)
- 2.FEV1: More Than a Measurement of Lung Function, A Biomarker of HealthSmith JP, Johnson KL, Williams AB, et al. J Clin Med 2023;12(4)
- 3.Mortality and cardiovascular and respiratory morbidity in individuals with impaired FEV1: An international, community-based cohort studyDuong M, Rangarajan S, Teo K, et al. Lancet Glob Health 2019;7(5)
- 4.All-cause and cardiovascular mortality in relation to lung function in the full range of distribution across four Eastern European cohortsCourt T, Capkova N, Pająk A, et al. Sci Rep 2022;12(1)
- 5.Association between lung function and mortality in middle-aged and older Chinese: A prospective cohort studyWang Y, Chen Y, Zhang Y, et al. Front Public Health 2022;10
- 6.Ageing and the border between health and diseaseMacNee W, Rabinovich RA, Choudhury G. Eur Respir J 2014;44(5)
- 7.Multi-ethnic reference values for spirometry for the 3-95-yr age range: The Global Lung Function 2012 EquationsQuanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MSM, Zheng J, Stocks J. Eur Respir J 2012;40(6)
- 8.Longitudinal decline in lung function: a community-based cohort study in KoreaLeem AY, Park B, Kim YS, Chang J, Won S, Jung JY. Sci Rep 2019;9(1)
- 9.Effect of Smoking on Lung Function Decline in a Retrospective Study of a Health Examination Population in Chinese MalesTian T, Jiang X, Qin R, Ding Y, Yu C, Xu X, Song C. Front Med 2023;9
- 10.Effects of Cigarette Smoking and Age on Pulmonary Function Tests in ≥ 40 Years Old Adults in JordanRawashdeh A, Alnawaiseh N. Biomed Pharmacol J 2018;11(2)
- 11.Get help stopping smokingReeth Medical Practice. Reeth Medical Practice 2024
- 12.Home spirometry appears accurate and feasible for monitoring chronic respiratory diseaseWilson CL, McLaughlin C, Cairncross A, Gabbay E, Noble PB, Blakey JD, Crawford AL. ERJ Open Res 2024;10(3)
- 13.ARTP statement on pulmonary function testing 2020Sylvester KP, Clayton N, Cliff I, Hepple M, Kendrick A, Kirkby J, et al. BMJ Open Respir Res 2020;7(1)
- 14.Technical validity and usability of a novel smartphone‐connected spirometry device for pediatric patients with asthma and cystic fibrosisKruizinga MD, Essers E, Stuurman FE, Zhuparris A, van Eik N, Janssens HM, et al. Pediatr Pulmonol 2020;55(9)
- 15.Home spirometry appears accurate and feasible for monitoring chronic respiratory diseaseWilson CL, McLaughlin C, Cairncross A, Gabbay E, Noble PB, Blakey JD, Crawford AL. ERJ Open Res 2024;10(3)
- 16.MTI gets FDA clearance for connected spirometerMack H. MobiHealthNews 2017
- 17.Alveofit receives FDA approval for portable digital spirometerMedical Device Network 2023
- 18.Chronic obstructive pulmonary diseaseAbadian Sharifabad M. BMJ Best Practice 2024
- 19.Longitudinal decline in lung function: a community-based cohort study in KoreaLeem AY, Park B, Kim YS, Chang J, Won S, Jung JY. Sci Rep 2019;9(1)
- 20.Airflow obstruction, lung function, and risk of incident heart failure: the Atherosclerosis Risk in Communities (ARIC) studyAgarwal SK, Heiss G, Barr RG, Chang PP, Loehr LR, Chambless LE, Shahar E, Kitzman DW, Rosamond WD. Eur J Heart Fail 2012;14(4)
- 21.Declining Lung Function and Cardiovascular Risk: The Atherosclerosis Risk in Communities (ARIC) StudySilvestre OM, Nadruz W Jr, Roca GQ, Claggett B, Solomon SD, Mirabelli MC, London SJ, Loehr LR, Shah AM. J Am Coll Cardiol 2018;72(10)
- 22.Lung function, insulin resistance and incidence of cardiovascular disease: a longitudinal cohort studyEngström G, Hedblad B, Nilsson P, Wollmer P, Berglund G, Janzon L. Diabet Med 2010;27(10)
- 23.Association between lung function and insulin resistance in elderly people: a cross-sectional analysis from the Pro.V.A. studyFimognari FL, Loffredo L, Di Tanna GL, Martino F, Loffredo S, Violi F. ERJ Open Res 2021;7(3)
- 24.NF-κB-dependent airway inflammation triggers systemic insulin resistanceCyphert TJ, Morris RT, House LM, Barnes TM, Otero YF, Barham WJ, Hunt RP, Zaynagetdinov R, Yull FE, Blackwell TS, McGuinness OP. Am J Physiol Regul Integr Comp Physiol 2015;309(9)
- 25.Inflammation and insulin resistanceShoelson SE, Lee J, Goldfine AB. J Clin Invest 2006;116(7)
- 26.Impaired Lung Function, Lung Disease, and Risk of Incident DementiaLutsey PL, Chen N, Mirabelli MC, Lakshminarayan K, Knopman DS, Vossel KA, Gottesman RF, Mosley TH, Alonso A. Am J Respir Crit Care Med 2019;199(11)
- 27.Impaired Lung Function, Lung Disease, and Risk of Incident DementiaLutsey PL, Chen N, Mirabelli MC, Lakshminarayan K, Knopman DS, Vossel KA, Gottesman RF, Mosley TH, Alonso A. Am J Respir Crit Care Med 2019;199(11)
- 28.Early Midlife Pulmonary Function and Dementia RiskGilsanz P, Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Alzheimer Dis Assoc Disord 2018;32(4)
- 29.Air pollution and brain health: an emerging public health concernChen JC, Wang X, Wellenius GA, Serre ML, Driscoll I, Casanova R, McArdle JJ, Manson JE, Chui HC, Espeland MA. Front Aging Neurosci 2024;16
- 30.Air pollution and brain health: an emerging public health concernChen JC, Wang X, Wellenius GA, Serre ML, Driscoll I, Casanova R, McArdle JJ, Manson JE, Chui HC, Espeland MA. Front Aging Neurosci 2024;16
- 31.Association of cognitive reserve with transitions across cognitive states and death in older adults: A 15-year follow-up studyLi Y, Dekhtyar S, Grande G, Kalpouzos G, Gregorio C, Laukka EJ, Qiu C. Alzheimers Dement 2024;20(7)
- 32.All-cause and cardiovascular mortality in relation to lung function in the full range of distribution across four Eastern European cohortsCourt T, Capkova N, Pająk A, Malyutina S, Tamosiunas A, Bobák M, Pikhart H. Sci Rep 2022;12(1)
- 33.Longitudinal decline in lung function: a community-based cohort study in KoreaLeem AY, Park B, Kim YS, Chang J, Won S, Jung JY. Sci Rep 2019;9(1)
- 34.Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort studyGarcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Am J Respir Crit Care Med 2007;175(5)
- 35.Effect of pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis of randomized controlled trialsZhang H, Hu D, Xu Y, Wu L, Lou L. Ann Med 2022;54(1)
- 36.DunedinPACE, a DNA methylation biomarker of the pace of agingBelsky DW, Caspi A, Corcoran DL, Sugden K, Poulton R, Arseneault L, Baccarelli A, Chamarti K, Gao X, Hannon E, Harrington HL, Houts R, Kothari M, Kwon D, Mill J, Schwartz J, Vokonas P, Wang C, Williams BS, Moffitt TE. eLife 2022;11(e73420)
- 37.TruDiagnostic Announces a New Multi-Omic Informed Biological Aging Clock that Outperforms Previous Age Calculating MethodsTruDiagnostic. PR Newswire 2023
- 38.SYMPHONYAge: A revolutionary new way of looking at agingTruDiagnostic. TruDiagnostic 2024
- 39.SYMPHONYAge Technical DocumentationTruDiagnostic. TruDiagnostic 2024
- 40.DunedinPACE: A DNA methylation biomarker of the pace of agingBelsky DW, Caspi A, Houts R, Cohen HJ, Corcoran DL, Danese A, Harrington H, Israel S, Levine ME, Schaefer JD, Sugden K, Williams B, Yashin AI, Poulton R, Moffitt TE. bioRxiv 2023